High School Chemistry: Homework Help Resource, College Chemistry: Homework Help Resource, ILTS Science - Chemistry (106): Test Practice and Study Guide, High School Physical Science: Homework Help Resource, High School Physical Science: Tutoring Solution, NY Regents Exam - Chemistry: Help and Review, Glencoe Chemistry - Matter And Change: Online Textbook Help, Intro to Physics for Teachers: Professional Development, All Teacher Certification Test Prep Courses, Working Scholars Bringing Tuition-Free College to the Community. This is just one example of the types of things that counting electrons can predict. Different isotopes of an element generally have the same physical and chemical properties due to the fact they have the same numbers of protons and electrons. The number of neutrons can be different, even in atoms of the same element. Mass number equals number of sub atomic particles in the nucleus. Conodonts are commonly found in limestone rocks as these creatures swam in the seas in which the limestone was deposited. The mass number (\(A\)) of an atom represents the sum of protons and neutrons in the nucleus of that atom and provides a very close approximation to the mass of the atom in atomic mass units. Explain what isotopes are and how an isotope affects an element's atomic mass. The chemical properties of the element don't change, but the atomic mass of the element is different if it has, say, 8 neutrons versus 9 neutrons. Invertebrate animals (those lacking a backbone) have been using dissolved calcite ions to build their shells since at least the Cambrian (~550 million years ago). Calcite and calcium carbonate are common on the Earth and in the oceans, and can take several forms. Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples. Some oxygen atoms have 9 neutrons, while others have 10 neutrons. To analyze stable isotopes of carbon and oxygen from speleothems, they are cut out of a cave and taken to a lab, where they are sawed in half and polished. changeable within natural constraints. Two of the most common stable isotopes that are used by geoscientists are those of carbon (C) and oxygen (O). 16 total protons and neutrons minus 8 protons leaves us with 8 neutrons. Electrons have a certain way of arranging themselves on atoms, and we can think of electrons as existing in "shells" around the atom's nucleus. Get access to this video and our entire Q&A library. One of the many ways in which paleoclimatologists know past climate and ocean conditions is by using the chemical makeup of rock and fossil specimens. In our scientific observations, oxygen has either 8, 9, or 10 neutrons. [3] Researchers need to avoid improper or prolonged storage of the samples for accurate measurements.[3]. Designed and developed by industry professionals for industry professionals. An isotope is an element that has a different number of neutrons in different atoms. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. They are just rarer.
 The atomic number tells you how many protons are in the atom's nucleus. Learn how and when to remove this template message, "The AME2016 atomic mass evaluation (II). Calcite and calcium carbonate are common on the Earth and in the oceans, and can take several forms. This is because the mass of the proton and neutron are each about 1 amu, while the mass of the electron is very small in comparison.
The atomic number tells you how many protons are in the atom's nucleus. Learn how and when to remove this template message, "The AME2016 atomic mass evaluation (II). Calcite and calcium carbonate are common on the Earth and in the oceans, and can take several forms. This is because the mass of the proton and neutron are each about 1 amu, while the mass of the electron is very small in comparison. 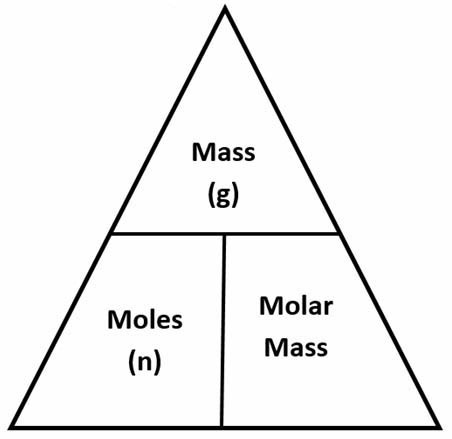 All other trademarks and copyrights are the property of their respective owners. We got the mass as #16# units, and so it'll have #16-8=8# neutrons. However, changing numbers of neutrons will result in isotopes of differing masses. Tables, graphs, and references", "Table of Isotopic Masses and Natural Abundances", "Oxidized silver cups can skew oxygen isotope results of small samples", https://en.wikipedia.org/w/index.php?title=Oxygen-16&oldid=1075735156, Articles needing additional references from February 2020, All articles needing additional references, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 7 March 2022, at 11:09. When using the metric system, it is helpful to know how to convert units from other systems. Chemical elements are found in different versions, called isotopes. depending on the reference point, oxygen can gain electrons during molecular bonding). Oxygen atoms with 9 neutrons would have a mass number of 17 (8 \(\mathrm p^+\) + 9 \(\mathrm n^0\)), meaning they would have a mass of about 17 amu. Oxygen-17 (nine neutrons) is also stable, but is rarer.
All other trademarks and copyrights are the property of their respective owners. We got the mass as #16# units, and so it'll have #16-8=8# neutrons. However, changing numbers of neutrons will result in isotopes of differing masses. Tables, graphs, and references", "Table of Isotopic Masses and Natural Abundances", "Oxidized silver cups can skew oxygen isotope results of small samples", https://en.wikipedia.org/w/index.php?title=Oxygen-16&oldid=1075735156, Articles needing additional references from February 2020, All articles needing additional references, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 7 March 2022, at 11:09. When using the metric system, it is helpful to know how to convert units from other systems. Chemical elements are found in different versions, called isotopes. depending on the reference point, oxygen can gain electrons during molecular bonding). Oxygen atoms with 9 neutrons would have a mass number of 17 (8 \(\mathrm p^+\) + 9 \(\mathrm n^0\)), meaning they would have a mass of about 17 amu. Oxygen-17 (nine neutrons) is also stable, but is rarer.
Oxygen atoms with 8 neutrons would have a mass number of 16 (8 \(\mathrm p^+\) + 8\(\mathrm n^0\)), meaning they would have a mass of about 16 amu. By "sharing" 4 of them, they each get to "keep" half of the remaining electrons (16 total - 4 shared = 12 remaining, 12/2 = 6 each). For example, there are three isotopes of the element oxygen (O): Oxygen 16, 17, and 18. Atomic number gives the number of protons, which equals the number of electrons. All rights reserved. Learn how fast light travels and find the speed of light in meters or miles per second. In addition, some protists, such as planktic and benthic foraminifera, use calcite to build their tests. Oxygen has several isotopes, and they have different numbers of neutrons. Since it is neutral, it also has eight protons! Next, lets consider the number of neutrons and electrons in oxygen: in short, theyre
While all carbon atoms have six protons and most have six neutrons, some carbon atoms have seven neutrons, while others have eight. Thanks for your question, and lets start with the basics: how chemical elements are
What are the two main types of chemical bonds? , as these are very common in paleoclimatology (especially to study our oceans), but will also briefly touch on other proxies used for isotope analyses. For a more detailed account of how mass spectrometry works, click here. Understand the significance of light-years and lookback time with examples. Most 16O is synthesized at the end of the helium fusion process in stars; the triple-alpha process creates 12C, which captures an additional 4He to make 16O. This means that a neutral oxygen atom will have 8 electrons (but this number can change! Instead, we use stable isotopes that are not undergoing radioactive decay. That means it has 8 protons and 8 electrons. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. This tells us that most carbon atoms have 6 protons and 6 neutrons, and some carbon atoms have 6 protons and maybe 7 or 8 neutrons. Positive and negative ions will attract each to form solids, some liquids, and some gases. There are several different types of mass spectrometers, but one of the common ways to measure isotopes is to manipulate them by magnets and electric fields, and shoot them down a bent tube. It does this in a clever way; it looks for a partner! The atomic nucleus contains both protons and neutrons, but the interesting thing is that there aren't always an equal number of neutrons and protons. A heavier ion/atom/molecule is harder for the magnet to deflect, so it will only turn slightly, while a lighter i/a/m has less inertia and is easier to turn. Elements have a charged balance (neither positive or negative) because they have an equal number of electrons and protons. How is the atomic mass number changed by gamma decay. The oxygen we breathe is not in atomic form. Which subatomic particles contribute to an Ionic bonds are electrostatic interactions between two oppositely charged ions. Although there are several types of stable isotopes, we will mainly talk about carbon and oxygen obtained from planktic and benthic foraminifera, as these are very common in paleoclimatology (especially to study our oceans), but will also briefly touch on other proxies used for isotope analyses. Compounds and molecules are built from elements composed of at least two atoms joined with a chemical bond. The situation with neutrons (which have no charge) can be a little more complicated, but the number of neutrons is often the same as or similar to the number of protons. Oxygen-16 (16O) is a stable isotope of oxygen, having 8 neutrons and 8 protons in its nucleus. Understand the characteristics of ideal gases and know examples of the properties of gases. This atom will have 10 neutrons as 18 - 8 = 10. Remember that chemical elements are composed of some number of protons, neutrons, and electrons. Chemical elements are found in different versions, called. Most atoms of oxygen also have 8 neutrons. Quite often, there are one or two isotopes of an element that are the most stable and common. Understand the definition of atomic mass, atomic number, and atomic weight. Each sample is loaded into a vial, and all the vials are then put into a carousel (see image at left, with red arrow pointing to sample carousel). Paleoclimatologists obtain carbon and oxygen isotopes from calcite, a common variety of calcium carbonate, with the chemical formula CaCO3. read it here. That number tells you the number of protons an element has, and by extension tells you the number of electrons it has. Approximately three drops of acid are put into the vials to dissolve the sample, creating a gas that contains the. Biology Laboratory | Terms of use. Calcite is also used by marine organisms to build their shells and hard parts. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Therefore, oxygen 16 has 8 protons and 8 neutrons, oxygen 17 has 8 protons and 9 neutrons, and oxygen 18 has 8 protons and 10 neutrons. The number of neutrons varies according to the isotope: the stable isotopes have 8, 9, or 10 neutrons(Wikipedia). The reason for this has to do with the number of electrons oxygen has. . As discussed in the the previous section, atoms of the same element always have the same number of protons. This is the O2 gas that you and I breathe! Each isotope of oxygen contains 8 protons, but differs in the number of neutrons. Ionic Bond Formation, Types & Examples | What is an Ionic Bond? In terms of mass, protons and neutrons have about the same mass, and electrons have very little mass in comparison. Many isotopes occur naturally. This information is sent to a computer, which gives the researcher data on the amount of each isotope in every sample. An isotope number is a shorthand representation of its mass. Thus, lighter molecules are deflected more than heavier ones. One fun thing people are doing is finding the element that is the same number as their age.
Some extant (still living) animals, like sea urchins and oysters also build their skeletons from calcite. Oxygen is atomic number 8 on the periodic table, which means it has 8 protons! An isotope number is a shorthand representation of its mass. [2] The relative and absolute abundance of 16O are high because it is a principal product of stellar evolution and because it is a primordial isotope, meaning it can be made by stars that were initially made exclusively of hydrogen. This calculation can get surprisingly tricky! Thus, oxygen tends to gain 2 electrons, for a total of 10 electrons. Covalent Bonds Formation & Examples | What is a Covalent Bond? Elements are charge neutral (meaning the charges add up to 0), and since protons have a positive charge, each proton is balanced out by 1 electron, which has a negative charge. FASTER Systems provides Court Accounting, Estate Tax and Gift Tax Software and Preparation Services to help todays trust and estate professional meet their compliance requirements. While the number of protons remains the same for all isotopes, otherwise the atom would change elements to one with a matching number of protons, the neutrons in the nucleus can change without changing the element. Consider oxygen, which has an atomic number (\(Z\)) of 8. Thats the long version of this simple fact: oxygen will always have 8 protons, whether on Mars, Pluto, Earth, yesterday, or the day after tomorrow. For oxygen, the atomic mass is 16 AMU (Atomic Mass Units). Types of Chemical Bonds: Ionic vs Covalent | Examples of Chemical Bonds. Find compounds which contain this structure, Find compounds which resemble this structure, European Molecular electrons by high energy collisions with particle accelerators, but thats more artificial, and To learn how paleoclimatologists interpret carbon and oxygen isotopes, continue to the Carbon & Oxygen Isotopes page! When a solid dissolves in water, the positive and negative ions break apart and dissociate through the water. For nearly all elements, this means gaining or losing electrons to other atoms, thus producing ions. Learn about chemical bonding, explore how hydrogen bonds form, discover the differences between intramolecular forces and intermolecular forces, then review an example of how these bonds are used. How do you find mass number of an isotope? This forms an aqueous (a water based) solution: In the above equation, the (s) indicates a solid material (table salt), whereas the (aq) indicates that these ions are dissolved in an aqueous solution. Common fossil groups that utilize calcite include brachiopods, trilobites, and ancient echinoderms, such as, . When Oxygen loses or gains neutrons it becomes an isotope. Oxygen has an atomic number of #8#, and the mass number of an atom is the sum of its atomic number plus its neutron number. For now we can think of atoms as having "inner" and "outer" shells. The positive charge of 1 proton exactly cancels the negative charge of 1 electron. Unlike the calcareous brachiopods and trilobites that they lived among, conodont teeth are made of apatite, or calcium phosphate, with the chemical formula Ca, Once the appropriate material (limestone samples, speleothems, or fossils) is collected for isotope analyses, a small sample is put into a, to measure the amounts of carbon and oxygen isotopes within each sample. Learn how to define acids and bases, explore the pH scale, and discover how to find pH values. Atom Overview, Structure & Examples | What is an Atom? What type of radioactive decay produces no change in mass number? Astronomy is the scientific study of celestial bodies in outer space. Approximately three drops of acid are put into the vials to dissolve the sample, creating a gas that contains the ions to be measured. Learn what the properties of gases are and discover the kinetic molecular theory of gases. What is atomic number, mass number and atomic mass? In addition, some protists, such as planktic and benthic, , use calcite to build their tests. Shells are filled from the innermost to the outermost, so since oxygen has 8 electrons, we put 2 in the inner shell, and the remaining 6 in the outer shell. Each isotope of oxygen contains 8 protons, but differs in the number of neutrons. Most oxygen atoms also have 8 neutrons, but it is possible for an oxygen atom to have 9 or 10 neutrons. 16 - 8 = 8, Oxygen exists in isotopic form and has an isotope with mass number 18. If all of the oxygen atoms we know about, always had 8 protons and 8 neutrons, then the atomic weight would be 16. One refinement is to consider ions, whereas the answers at the linked question considers only neutral oxygen atoms. When this happens, the elements become. Write the different isotope notations or isotope symbols for this isotope. The stable isotope of oxygen with relative atomic mass 15.994914. What does the difference between the mass number and the atomic number tell us? Understand the definition of covalent bonds and how they are formed. Because protons and neutrons are roughly equal in mass, an isotopes number is equal to the sum of its protons and neutrons. Your question is an interesting one, because it is often the starting point for many other questions we can ask about elements, chemicals, and materials. Oxygen atoms with 10 neutrons would have a mass number of 18 (8 \(\mathrm p^+\) + 10 \(\mathrm n^0\)), meaning they would have a mass of about 18 amu. This means that oxygen atoms have 8 protons. This identifies the atom. Explore atoms. This means that it has eight electrons in its neutral state. Therefore, oxygen 16 has 8 protons and 8 neutrons, oxygen 17 has 8 protons and 9 neutrons, and oxygen 18 has 8 protons and 10 neutrons. There are two main types of isotopes that geoscientists use to interpret the ancient Earth: stable and unstable isotopes. Know its formula and learn how to compute it through given examples. Oxygen is the eighth element in the periodic table, with the symbol O. There are several different types of mass spectrometers, but one of the common ways to measure isotopes is to manipulate them by magnets and electric fields, and shoot them down a bent tube. See the concept mass number vs atomic mass. Here's where it gets advanced and cool! For a video demonstration on how ions are deflected within a mass spectrometer, click here. look up how common an isotope is. Atoms of the same element that containthe same number of protons, but different numbers of neutrons, are known as. Often, the number of electrons in an element can tell us about the what color it might be, whether or not it's a magnet, or what types of other elements it will react with. The periodic table tells us information about every element in existence in its neutral state. Because rainwater is slightly acidic, prolonged exposure to rain will chemically erode away limestone rock formations (or even a limestone statue for that matter). Both of these isotopes are stable (i.e. Explore how to draw the Bohr model of hydrogen and argon, given their electron shells. Several radioactive isotopes occur naturally, and not all are bad or cause harm to humans. Mass number is the total count of both protons and neutrons in an atom. Learn how ionic bonds are formed and what holds ionic compounds together. Two of the most common stable isotopes that are used by geoscientists are those of, . to be measured. This service is an Elixir Core Data Resource. This isotope-related article is a stub. Oxygen atoms with a mass number of 16 are called oxygen-16, or O-16, while oxygen atoms with a mass number of 17 are called oxygen-17, or O-17, and oxygen atoms with a mass number of 18 are called oxygen-18, or O-18. The mass number of oxygen-16 is 16. Common fossil groups that utilize calcite include brachiopods, trilobites, and ancient echinoderms, such as blastoids. Protons and neutrons are found in the nucleus, so the number of neutrons is found by subtracting the proton number from the mass number I.e. Looking at the periodic table, oxygen has atomic number 8 and atomic weight 15.999.
defined, especially on the periodic table of elements. Calcium, sodium, magnesium and potassium are sometimes included as macronutrients because they are required in relatively large quantities compared with other vitamins and minerals. The atomic number is 80, so there are 80 p, Since the atom is neutral, there are also 80 e. Starting with \(\text{mass number}\:(A)=\text{number of protons}+\text{number of neutrons}\), rearrange the equation to solve for the number of neutrons.
In this formula, there are three elements: calcium (Ca), carbon (C), and three oxygen atoms (O). Now, in terms of electrons, oxygen should contain 8 electrons in order to be isoelectric, which is a way of saying the electrons equal the number of protons, rendering no charge to the oxygen atom, and this is considered the most natural state of the oxygen atom (you can add/subtract probably seen one in your science instructors room) determine the element and it refers to the number of protons as a definitional part of the elements identity. Most carbon atoms have 6 protons and 6 neutrons, so the atomic mass is very close to 12 AMU. This entity has been manually annotated by the ChEBI Team. Remember that chemical elements are composed of some number of protons, neutrons, and electrons. ).
However, paleoclimatologists do not commonly work with these unstable isotopes. These scientists can analyze conodonts to obtain oxygen isotopes. This is answered pretty completely on ScienceLine here.